Upcoming Catalysts (Xeris)
- Opinions
- Upcoming Catalysts (Xeris)

Since many people ask about upcoming catalysts, I’ve decided to put together a few things that are on the horizon. A lot of the information can be found in the ER transcripts, but I know there are a lot of lazy investors who will never read an ER transcript so I’ll do my best to help them out. Please note that these are catalysts for Xeris only and not potential catalyst from Strongbridge.
Post-Bariatric Hypoglycemia (PBH):
According to to Paul they are expecting a Type C meeting in early July with the FDA on their PBH, post-bariatric hypoglycemia, clinical program. I wouldn’t expect to hear anything from this meeting until early August, end of July at best.
“Given the scope of the questions and the dialogue we’ve had with the FDA, they’ve advised us to submit a formal meeting request to discuss these points further. We’ve done that and anticipate a Type C meeting to be held in early July.”
Paul Edick – Q1 2021 Earnings Call
Exercise-Induced Hyperglycemia (EIH):
The EIH clinical study is not looking too promising and I would expect them to push this study to the back burner. On the last ER call Paul says the FDA wants two big studies done and the scope and cost is not something Xeris wants to take on.
“Last week, we received additional written feedback from the FDA. The bottomline is that in order to pursue a prevention indication, they’re now asking us to do two very large double-blind placebo-controlled studies with at least one-year of follow up. The size and scope of such an extensive clinical program for Xeris at this time would be too costly for us to undertake.”
Paul Edick – Q1 2021 Earnings Call
He does say they are “evaluating alternative clinical approaches”, so it’s still possible they could find a path with the FDA for moving forward, but I wouldn’t expect any news in the near future on EIH.
Pramlintide-Insulin:
Xeris has submitted a proposed Phase 3 study for the pramlintied-insulin co-formulation program, and they anticipate a response sometime in the third quarter. Based off this I would expect an update in early fourth quarter, late October or early November, on the results of that meeting. Remember once they get approval for their Phase 3, they do not plan on moving forward alone. So until they have a partner for this program don’t expect there to be much change in the stock price.
“We submitted the request for follow-up meetings for further clarification and anticipate a response in the third quarter. Based on that feedback, we will initiate a process to out license the program as we’ve mentioned previously…”
Paul Edick – Q1 2021 Earnings Call
Diazepam:
Xeris has been looking for an out license partner for Diazepam and nobody knows when/if they will find a partner so it’s still a potential upcoming catalyst, but no way to predict a timeline.
Ogluo:
Xeris plans to launch Ogluo by the fourth quarter in the EU and UK. On the recent Jefferies Conference, Paul mentioned they were in talks with a partner number of people for Ogluo and were in the “data room” (Source @ 25:38). Based off the time it would take to launch by fourth quarter in the EU and UK, personally I expect an Olguo partnership announcement by end of June, at the earliest, and no later than end of July.
Gvoke Sales:
“..we’re expecting a more normal back-to-school in 2021 than what we’ve saw in 2020, more reflective of what you might have seen in 2018 and 2019..”
Paul Edick – Q1 2021 Earnings Call
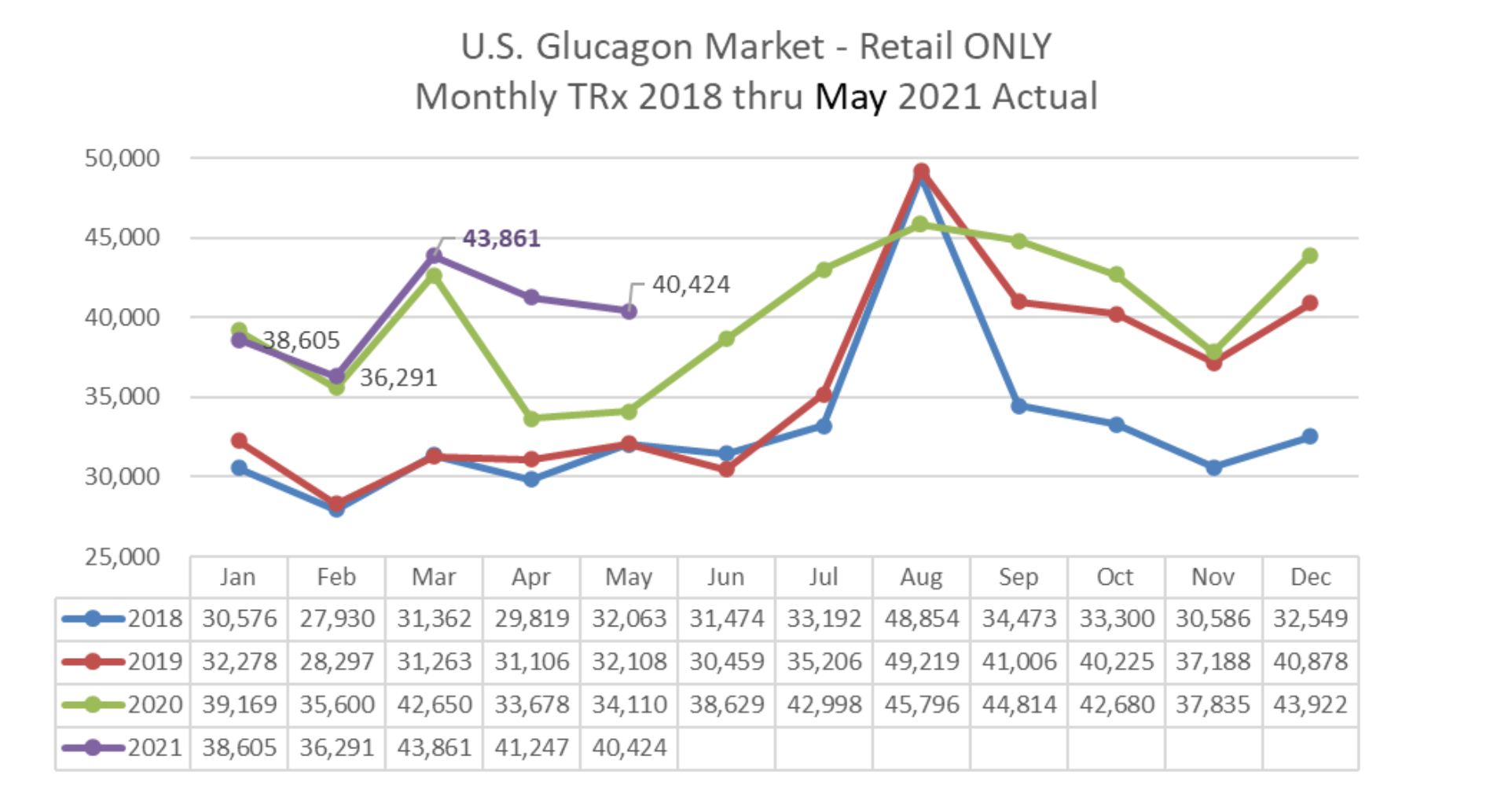
With the pandemic in 2020 the glucagon market did not see the typical back-to-school increase in sales that they have normally seen in the past. In terms of sales, this years back-to-school should be more reflective of 2018 and 2019, where sales spiked. And remember that Gvoke was launched in July 2020 so Gvoke has not seen a normal “back-to-school” event yet.
Disclaimer- I am not a financial advisor and this is not financial advice.
Updates:
- Gvoke Sales chart updated 6/22 with chart from June 2021 presentation